The inward rectifying K+ channels (IRKs) are common ion channels that encompass seven distinct subtypes, each a potential target for pathology or a potential actor in various insults to the nervous system, the retina included. IRKs in the retina are found in Müller glia, retinal pigment epithelium and on neurons in retina, so are fundamental to retinal physiology. However, their function and mechanisms of action have largely been unknown.
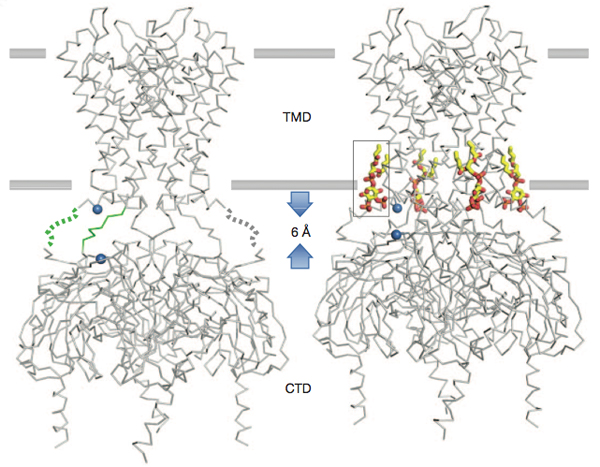
This paper by Scott B. Hansen, Xiao Tao and Roderick MacKinnon identifies the X-ray crystal structure of a Kir2.2 channel in complex with phosphatidylinositol 4,5-bisphosphate (PIP2), a common regulator of a large number of ion channels and the principle agonist for the Kir2 channels. Furthermore, this paper reveals a potential mechanism of action for this channel that involves a phospholipid binding domain in the trans-membrane region for PIP2 that specifically controls the resting membrane potential. This ion channel/receptor/ligand interaction then subsequently induces a substantial conformational change similar to those observed in other ion channels found at synapses, which in retrospect makes sense, but until now was unknown. Pretty exciting work.