This paper is a collaboration of Dr. Wolfgang Baehr and two experts in guanylate cyclase enzymology, Dr. Alexander Dizhoor, and Dr. Kris Palczewski representing a long standing collaboration that has generated over fifty papers going back to 1994.
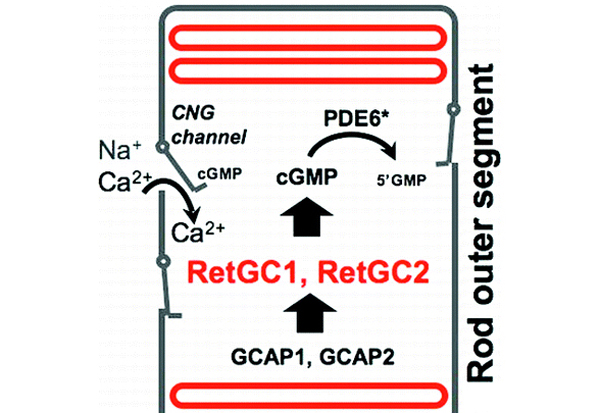
The results are based on animal models generated at the University of Utah and elucidate the contribution of the two guanlyate cyclases expressed in photoreceptors to the recovery of the dark state. Mouse photoreceptor function and survival critically depend on Ca(2+)-regulated retinal membrane guanylyl cyclase (RetGC), comprised of two isozymes, RetGC1 and RetGC2. This work characterized the content, catalytic constants, and regulation of native RetGC1 and RetGC2 isozymes using mice lacking guanylyl cyclase activating proteins GCAP1 and GCAP2 and deficient for either GUCY2F or GUCY2E genes, respectively. Interestingly, they found that the characteristics of both native RetGC isozymes were considerably different from other reported estimates made for mammalian RetGCs. Specifically, the content of RetGC1 per mouse rod outer segment(ROS) was at least 3-fold lower, the molar ratio (RetGC2:RetGC1) 6-fold higher, and the catalytic constants of both GCAP-activated isozymes between 12- and 19-fold higher than previously measured in bovine ROS.
Furthermore, the native RetGC isozymes had different basal activity and were accelerated 5-28-fold at physiological concentrations of GCAPs. RetGC2 alone was capable of contributing as much as 135-165 μM cGMP s(-1) or almost 23-28% to the maximal cGMP synthesis rate in mouse ROS. At the maximal level of activation by GCAP, this isozyme alone could provide a significantly high rate of cGMP synthesis compared to what is expected for normal recovery of a mouse rod, potentially helping to explain some of the unresolved paradoxes of rod physiology. GCAP-activated native RetGC1 and RetGC2 were less sensitive to inhibition by Ca(2+) in the presence of GCAP1 (EC(50Ca) ∼132-139 nM) than GCAP2 (EC(50Ca) ∼50-59 nM), thus arguing that Ca(2+) sensor properties of GCAP in a functional RetGC/GCAP complex are defined not by a particular target isozyme but the intrinsic properties of GCAPs themselves.