This abstract was presented today, Monday, April 30th at the 2018 Association for Research in Vision and Opthalmology (ARVO) meetings in Honolulu, Hawaii by Felix R. Vazquez-Chona, Tam T.T. Phuong, Oleg Yarishkin, myself (Bryan W. Jones), and David Krizaj
Purpose:
Loss of vision in diabetic retinopathy is associated with extensive shifts in retinal metabolic and synaptic function yet the general principles that govern the metabolic remodeling remain unknown. To define the metabolic signature in hyperglycemic retina we took advantage of in situ metabolomics, excitation mapping and gene knockdown. Specifically, we investigated whether manipulation of the swelling-activated calcium-permeable TRPV4 (transient receptor potential isoform 4) channel contributes to the metabolic program of the degenerating neurogliovascular subunit in diabetic mice.
Methods:
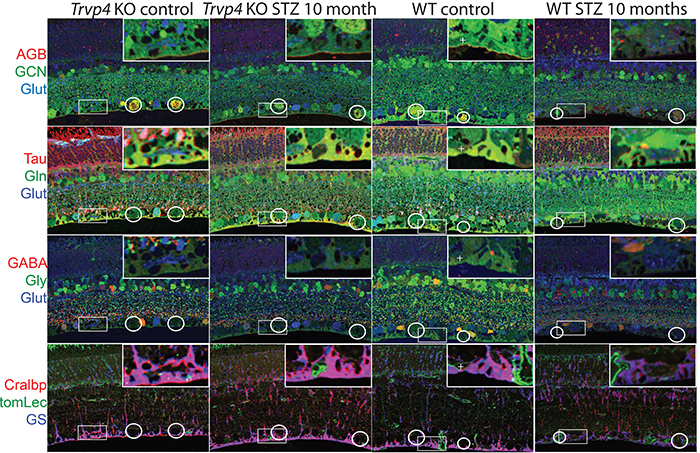
Type I diabetes in wild type (WT) and TRPV4-/- mice was induced with streptozotocin (STZ). We visualized glutamate (NMDA)-gated excitation and glucose transport using the organic cation agmatine (AGB2+) and the glucose analog glucosamine (GCN). Retinas were fixed in glutaraldehyde, sectioned, and incubated with antibodies targeting GCN and AGB. Cell classification and metabolic status were interrogated using Computational Metabolic Profiling (CMP) and probes against ADP, alanine, arginine, aspartate, citrulline, GABA, glutamate, glycine, glutathione, glutamine, isoleucine, taurine, glutamine synthetase, CRALBP, GFAP, and tomato lectin.
Results:
Amacrine and ganglion cells in control retinas responded to NMDA activation with a large elevations in AGB and GCN signals. Diabetic amacrine cells maintained a robust dynamic range of AGB and GCN signals which however were markedly diminished in RGCs. Metabolomic maps of diabetic WT retinas showed that the outer retina remains metabolically quiescent whereas the ganglion cell layer displayed cells with lower glutamate and GABA signals. Diabetic TRPV4-deficient retinas displayed metabolomic, excitation, and glucose transport maps that were comparable to control retinas. ERG analysis showed modest STZ-induced changes in scotopic a- and b-waves of WT and KO eyes.
Conclusions:
Our preliminary electrophysiological and metabolomic findings suggest that STZ-induced diabetes spares the inner retina but alters amacrine-ganglion cell signaling, the neurogliovascular unit organization together with RGC metabolism. TRPV4 inactivation partially rescues the metabolic, excitation, physiologic phenotypes imposed by hyperglycemia. These results suggest that ambient sensing through polymodal TRP channels links retinal neuronal, glial and endothelial signaling to cellular metabolism and visual function.