This abstract was presented today, May 5th at the 2015 Association for Research in Vision and Opthalmology (ARVO) meetings in Denver, Colorado by Karl Wahlin, Catherine Kim, Julien Maruotti, Srinivas Sripathi, Vinod Ranganathan, Kiara Eldred, myself, Robert Johnston, Cindy Berlinicke, and Donald Zack.
Purpose: Retinal degenerative diseases, such retinitis pigmentosa (RP), cause photoreceptor (PR) cell death and blindness. There are no cures and significant gaps exist in our understanding of how PR loss occurs. To address this we have developed genetically modified human induced pluripotent stem cell (hiPSC) retinal cell-reporters to investigate pathways promoting retinal differentiation and potentially uncover pathways leading to PR survival in human 3D disease models.
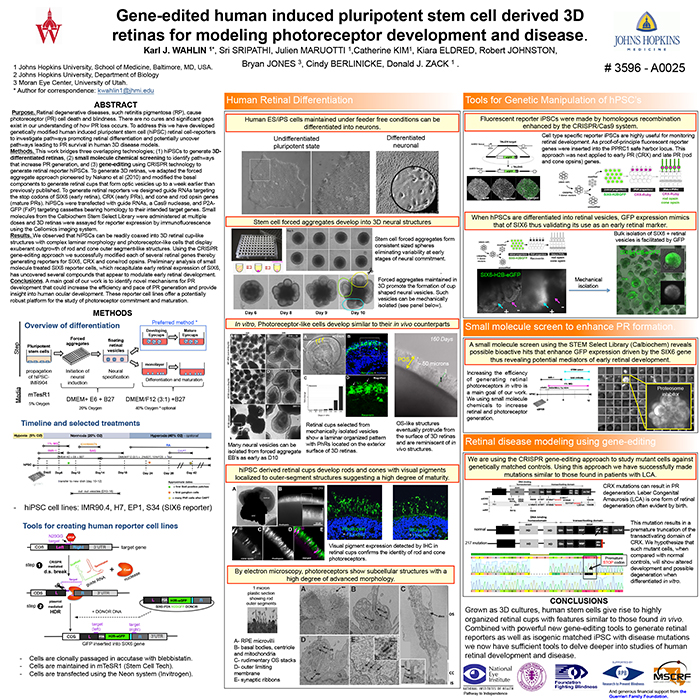
Methods: This work bridges three overlapping technologies; (1) hiPSCs to generate 3D-differentiated retinas, (2) small molecule chemical screening to identify pathways that increase PR generation, and (3) gene-editing using CRISPR technology to generate retinal reporter hiPSCs. To generate 3D retinas, we adapted the forced aggregate approach pioneered by Nakano et al (2010) and modified the basal components to generate retinal cups that form optic vesicles up to a week earlier than previously published. To generate retinal reporters we designed guide RNAs targeting the stop codons of SIX6 (early retina), CRX (early PRs), and cone and rod opsin genes (mature PRs). hiPSCs were transfected with guide RNAs, a Cas9 nuclease, and P2A-GFP (FxP) targeting cassettes bearing homology to their intended target genes. Small molecules from the Calbiochem Stem Select Library were administered at multiple doses and 3D retinas were assayed for reporter expression by immunofluorescence using the Cellomics imaging system.
Results: We observed that hiPSCs can be readily coaxed into 3D retinal cup-like structures with complex laminar morphology and photoreceptor-like cells that display exuberant outgrowth of rod and cone outer segment-like structures. Using the CRISPR gene-editing approach we successfully modified each of several retinal genes thereby generating reporters for SIX6, CRX and cone/rod opsins. Preliminary analysis of small molecule treated SIX6 reporter cells, which recapitulate early retinal expression of SIX6, has uncovered several compounds that appear to modulate early retinal development.
Conclusions: A main goal of our work is to identify novel mechanisms for PR development that could increase the efficiency and pace of PR generation and provide insight into human ocular development. These reporter cell lines offer a potentially robust platform for the study of photoreceptor commitment and maturation.
Layman Abstract: Retinal degenerative diseases, such retinitis pigmentosa (RP), cause dysfunction and death of photoreceptor (PR) cells leading to blindness. For most retinal degenerations there is no cure and significant gaps exist in our understanding of how PR loss occurs. Using CRISPR gene-editing technology we have developed genetically modified human induced pluripotent stem cell (hiPSC) retinal cell-reporters and retinal degeneration (RD)-associated hiPSCs that we are using to generate 3D-differentiated retinas. These hiPSCs can be coaxed into becoming retinal eyecup-like structures with complex laminar morphology and photoreceptor-like cells that display exuberant outgrowth of rod and cone outer segment-like structures, hallmark features of mature neural retinas. Investigating retinal development through administration of small molecule chemicals has led to the discovery of several potential candidates that modulate early retinal development. A main goal of our work is to identify novel mechanisms for retinal and PR development that could increase the efficiency and pace of PR generation and provide insight into human ocular development.