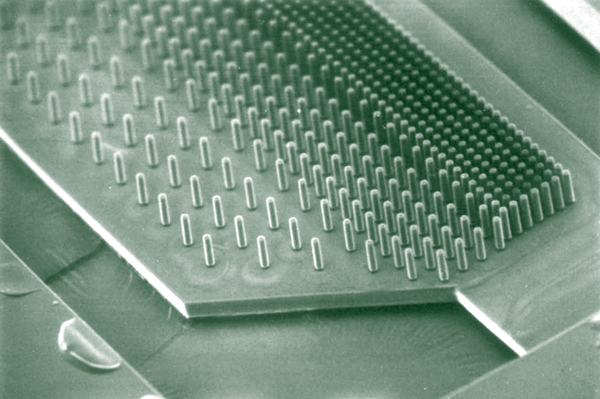
This image is of a retinal bionic pillar array that I am evaluating for some collaborators at Stanford who are developing a bionic retinal implant. Phil Huie and Daniel Palanker invited me out last summer to talk about retinal remodeling. That visit developed into a friendship and collaboration that hopefully will give some better insight into how to design retinal bionic implants given the current strategies and limitations imposed by the biology and engineering which are preventing successful implementation.
So, while we are talking about science, H (my wife) and I are sitting at the table over lunch last weekend while I am going through the last couple of Science issues. I was reading to her about a pretty cool special collection issue containing an article by Justin Sonnenburg, who is apparently doing a postdoc at Washington University St. Louis. His paper focuses on Bacteroides thetaiotaomicron in the gut and their role in consuming polysaccharides. These bacteria typically eat plant polysaccharides until they are gone at which point they then turn to mucopolysaccharide secretions to supplement their energy supply. The scientifically important thing about this study is that it nicely demonstrates that metabolic activities are altered according to substrate availability, thus my interest. H then says “why can’t these bacteria be used to treat patients with mucopolysaccharidosis (like Hunter disease)?” I think to myself “……………..Ummm,………….. damn, ………ummmm…..good question”, I had to think a bit before I answered. Based on my admittedly limited knowledge of genetic engineering and bacteriology, I finally had to say “Good question!” I don’t know, but perhaps she is on to something and “bacterial therapy” is an option that has been overlooked? The polysaccharide diseases like Hunter disease are metabolic disorders whose mechanisms of disease result from (typically) missing or malfunctioning/improperly assembled protiens. In the case of Hunter disease, these kids are missing an enzyme called iduronate sulphate sulphatase that cuts up mucopolysaccharides. If not broken down, these molecules build up causing cellular stress and oxidative damage. Could Bacteroides thetaiotaomicron be engineered to be non-pathogenic? Perhaps given they are part of the normal flora, this is not even a consideration? Bacterial therapy with Bacteroides thetaiotaomicron might help these kids out by exploiting their ability to consume polysaccharides in the gut thus reducing the amounts of polysaccharides that are transported across the intestinal wall. This would not be a cure per se, but it could be an additional therapy of sorts. Why not?