I gave a platform talk at this years ARVO. The abstract is below:
B. W. Jones, C. K. Chen, C. B. Watt, J. M. Frederick, W. Huang, W. Baehr, R. E. Marc. Ophthalmology, Univ Utah/Moran Eye Center, Salt Lake City, UT;
Purpose:
Our goal was to test the hypothesis that the neural retina is spared in rod degenerations. Computational molecular phenotyping (CMP) of human retinis pigmentosa (RP) specimens suggests that extensive neuronal and glial remodeling is triggered after rod depletion (Marc et al. 2001 IOVS 42: S117). Transgenic models of murine rod degeneration with different rates of rod loss (the GHL rhodopsin mutant, the RhoΔCT truncation mutant, and the RGS9 truncation mutant TG9N) were explored by CMP to track the fates of all retinal cell types.
Methods:
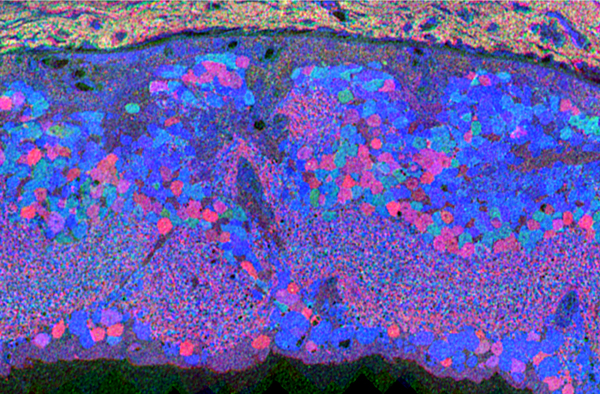
Retinas from animals 30-700+ post-natal days (PND) in age were glutaraldehyde fixed, assembled into tissue arrays, ultrathin-sectioned into sample arrays, probed for L-ala, L-asp, L-glu, L-gln, glutathione, taurine, GABA, rhodopsin and tyrosine hydroxylase signals, and analyzed by CMP to track cellular molecular signatures. Transgenic mice were compared to littermate controls.
Results:
Though neural remodeling of these mutant retinas progresses at different rates, all display similar sequelae following demise of the rods. TG9N bipolar cell layer thinning becomes apparent at PND 150 with mild changes in Muller cell morphology. Many bipolar cells have died by PND 365 and ectopic amacrine cells migrate into the ganglion cell layer. Muller cell hypertrophy becomes common and the inner plexiform layer erupts into the remnant sub-retinal space (SRS) and ganglion cell layer. Both GABA+ and glycine+ amacrine cells elaborate thousands of ectopic microneuromas into the remnant SRS. Whether these compact inhibitory neuron arbors form synaptic contacts or receive any excitatory drive is not yet known. Beyond twelve months, the TG9N neural retina continues to remodel with apparent ongoing ganglion cell loss and migration into distal retina. The GHL retina requires some 500-700 PND to become similarly disordered. Survivor neurons display anomalous elevated amino acid signatures.
Conclusion:
The TG9N, RhoΔCT and GHL models exhibit changes paralleling those of advanced human RP retinas: metabolic aberrations, neuronal cell loss and, perhaps more seriously, pathologic and likely dysfunctional ectopic patches of neuropil. The concept that the neural retina is largely preserved and experiences only minor changes after photoreceptor loss is incorrect. Rod degeneration is equivalent to deafferentation and triggers a cascade of defects perhaps inimical to simple rescue.
B.W. Jones, None; C.K. Chen, None; C.B. Watt, None; J.M. Frederick, None; W. Huang, None; W. Baehr, None; R.E. Marc, Signature Immunologics Inc E.